How Many Nonpolar Bonds Does Ch3oh Contain
Physical Chemistry Thermodynamics Structure and Change 10th ed Peter Atkins Julio de Paula 2014. Which of the following types of reactions is NOT enabled by breaking an alcohol CO bond.

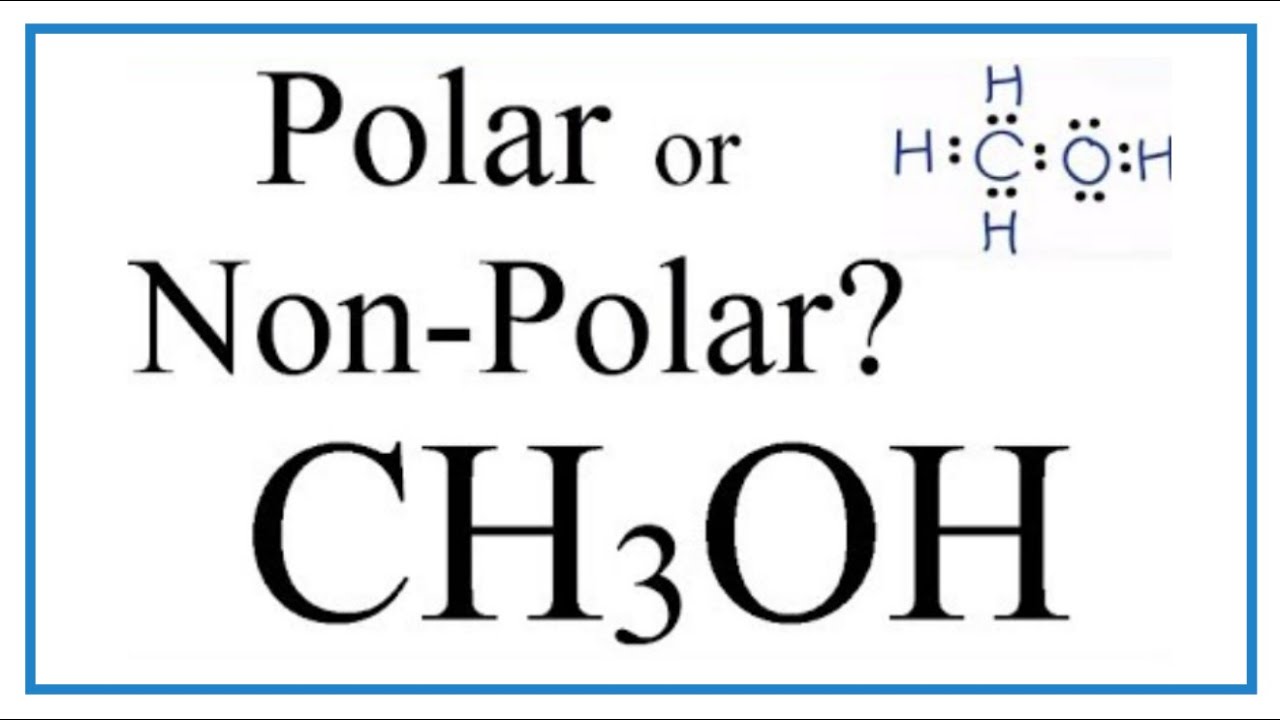
Ch3oh Lewis Structure Geometry Hybridization And Polarity Techiescientist
Which one of the following is most likely to be a covalent compound.

. Some examples of polar molecules which can. The structures we use to represent covalent compounds such as H2 and F2 are called Lewis structures. A CsOH B NF3 C.
Molecules which are capable of hydrogen bonds have hydrogen atoms which are covalently bonded to highly electronegative elements O N F. This concerted CH bond cleavage and the formation of CO and OH bonds is dependent on the alignment of the reacting partners. The presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar.
Covalent compounds are compounds that contain only covalent bonds. The relative stability of the hydroxide ion OH allows alcohols to undergo what. The transition state 11a leads to protonated.
Fill in the correct atoms or groups to complete the structure of the oxidation of 2-pentanol below. In 11a the reacting O 2 is aligned parallely with the CH bond of 1a resulting in a tight four-membered complex. A KF B CaCl2 C SF4 D Al2O3 E CaSO4.
Which one of the following is most likely to be a covalent compound. Molecular Cell Biology 5th ed - Lodish et al. O 2 being a nonpolar gas is less soluble in any solvent and.
This means the molecules will be soluble in a polar solvent such as water.
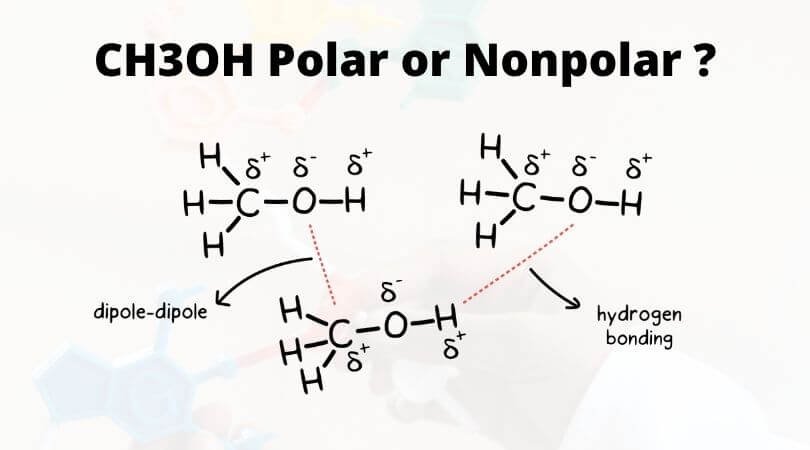
Ch3oh Polar Or Nonpolar Methanol Polarity Geometry Of Molecules

Ch3oh Lewis Structure Geometry Hybridization And Polarity Techiescientist
Comments
Post a Comment